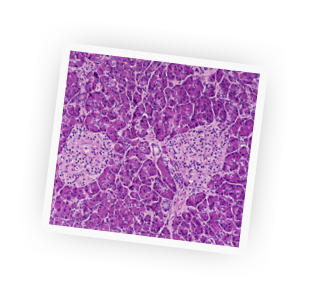
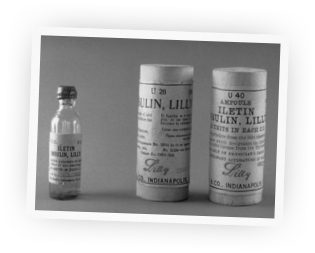
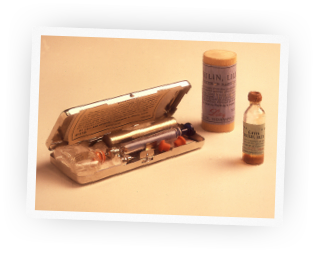
Celebrating the First Century of the World's First Life-Saving Drug
Before the discovery of insulin, people diagnosed with diabetes were often treated with severely restricted diets for months until they eventually died from the condition. But in 1920, something incredible happened that would change the lives of thousands of suffering people, and millions more over time.